|
Abstract: |
Introduction
For women between the ages of 40–79 years, breast cancer is the leading cause of all cancer-related deaths. The American National Cancer Institute estimates that approximately 2.4 million women with a history of breast cancer were alive in 2004 [1]. Approximately 10% of patients with breast cancer have metastatic disease from diagnosis and another 10–20% also have locally advanced breast cancer. In addition, up to one-third of early breast cancer cases will develop metastatic disease in the course of their lives [2].
Improvements in the outcome of metastatic breast cancer (MBC) have been observed in the last 30 years, but the overall prognosis remains poor with a median survival of 2–3 years [3]. A small percentage of MBC patients (2–5%) achieve complete response and maintain it for a long period of time [4].
There is a greater understanding of breast cancer heterogeneity and the availability of targeted therapies. The adequate treatment of MBC should be based on the therapeutic efficacy of the drug counter balanced by its toxicity profile, aiming at preserving/improving quality of life and the survival of the patient. Metastatic breast cancer therapeutic options can be divided according to tumour characteristics: hormone receptor-positive tumours can be treated with endocrine therapies, HER2-positive tumours can be treated with anti-HER2 agents, and chemotherapy can be used for treating MBC patients regardless of hormone receptors and HER2.
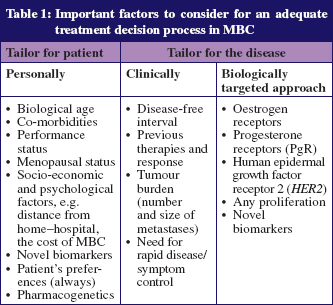
The treatment of MBC remains a challenge and several factors should be taken into consideration for the treatment decision process, as detailed in Table 1. A balance between treatment response and quality of life should be pursued, as the great majority of MBC patients remain incurable. The possibility of offering multiple lines of treatment to a particular patient makes the treatment decision process a difficult task. Although new therapeutic modalities are being developed, few randomised phase III studies evaluate these new drugs as a third-(or more) line of treatment [2, 5].
Chemotherapy for metastatic breast cancer
The response rate to first-line chemotherapy in MBC ranges from 30–70%, with an associated time to tumour progression (TTP) of 7–10 months. Treatment of MBC after progression to first-line treatment is associated with a lower response rate (20–30%) and subsequent worse TTP in the range of six months [6].
Anthracyclines and taxanes (docetaxel and paclitaxel) are the preferred options for first- and second-line chemotherapy in MBC if not already received in the adjuvant setting. In a recently conducted meta-analysis, response rate to the first-line treatment with single agent taxane or anthracycline was similar (38% and 33% respectively; p = 0.08) [7].
Approved chemotherapeutic options for patients progressing after anthracyclines and taxanes remain limited. Capecitabine is an approved FDA chemotherapeutic agent for treating MBC patients resistant to anthracyclines and taxanes. An analysis of 162 patients treated with capecitabine demonstrated a response rate of 20% and a progression-free survival (PFS) of 93 days, leading to capecitabine approval by the FDA [8]. Different studies confirmed the activity of capecitabine for second- and third-line setting with response rates varying from 15–29% [9–12]. Capecitabine also has a favourable toxicity profile with hand–foot syndrome being the most important side effect. Additionally, capecitabine is administered orally, which makes it a convenient option for MBC patients.
There is no standard therapeutic option for patients progressing after anthracycline, taxane and capecitabine (triple resistant group), although several agents are available. A phase II study conducted to evaluate the activity of ixabepilone in the triple resistant group demonstrated an overall response rate of 11.5% among 126 patients [13]. Very recently, eribulin, a new anti-microtubule agent with a unique mechanism of action of tubulin interaction, has provided a three-month improvement in overall survival in multi-treated (median four previous lines) MBC patients [14].
One controversial issue is whether combination chemotherapy (one or more drugs) should be preferred to sequential drug administration for the first-line treatment of MBC [15]. Recently, the European School of Oncology published the recommendation that chemotherapy given in a sequential monotherapy strategy should be the preferred choice in advanced disease, while combination of two cytotoxic agents should be reserved for cases with rapid disease progression, life-threatening metastases or the need for rapid symptom/disease control. It is important to highlight that chemotherapy combined with targeted agents was not addressed in this recommendation.
The major concerns in terms of toxicity for patients being treated with chemotherapy are febrile neutropenia, diarrhoea, skin toxicity, neuropathy, and cardiotoxicity for those treated with anthracyclines.
Hormonal therapy for metastatic breast cancer
The positivity of oestrogen and progesterone receptor (ER and PgR) in breast cancer can be considered one of the most important predictive factors for therapy response in clinical oncology. Up to 70% of patients with hormone receptor-positive breast cancer respond to hormonal therapy.
The available therapeutic options for hormone receptor-positive MBC are divided according to the menopausal status. Premenopausal patients can be initially treated with selective oestrogen receptor modulators (SERMs) such as tamoxifen and toremifene, ovarian ablation (oophorectomy or radiotherapy) or suppression (gonadotropin releasing hormone agonists) or a combined strategy (tamoxifen and ovarian suppression/ablation). SERMs act as a competitive antagonist of oestrogen for binding to ER. Tamoxifen, in the first-line setting, is associated with a response rate in the range of 50% and a long time to progression of 12–18 months [16]. In a meta-analysis of four randomised clinical trials (n = 506 patients) and a median follow-up of 6.8 years, the combined strategy was superior in terms of response rate, time progression and overall survival [17].
There are more initial treatment options for postmenopausal patients compared to premenopausal patients. The superiority of aromatase inhibitors (AIs) over tamoxifen guides most physicians to use this class of drugs in the first-line setting. In a meta-analysis of 23 clinical trials, superior response rate, TTP and overall survival favoured AIs over tamoxifen for the first-line treatment of MBC [18]. Data on the role of polymorphisms such as CYP2D6 on the activity of tamoxifen are still controversial and therefore it should not be used for treatment decision-making in clinical practice.
AIs suppress oestrogen levels in postmenopausal women by inhibiting or inactivating aromatase, an enzyme responsible for the synthesis of oestrogens from androgenic substrates. No agonistic effect is observed with AIs, but the oestrogen deprivation is associated with adverse events such as musculoskeletal pain, osteoporosis and vaginal dryness. Upon progression with AIs, different therapeutic options are available although no standard treatment exists. Fulvestrant and exemestane, a steroidal AI, were equally effective in a randomised clinical trial involving patients who were previously treated with a non-steroidal AI [19]. Fulvestrant is an ER antagonist that downregulates ER, and is given as intramuscular monthly injections. Tamoxifen is also a good therapeutic option. Additional palliative hormonal therapy options are progestatives and more recently low dose oestrogens [20, 21].
HER2 targeted therapy for metastatic breast cancer
The identification of the HER2 represents an important milestone in the field of targeted therapy in breast cancer. Patients with HER2-positive MBC should be treated with anti-HER2 therapy unless contraindicated. Trastuzumab is a monoclonal antibody designed to block the HER2 receptor and is used in combination with chemotherapy as the standard first-line treatment of HER2-positive MBC. In a randomised clinical trial, the addition of trastuzumab to paclitaxel increased overall response rate (16% vs 38%), median TTP (3.0 vs 6.9 months) and overall survival (18.4 vs 22.1 months) [22]. In a subsequent phase III trial, 399 HER2-positive, locally advanced or MBC patients who had progressed after prior anthracycline, taxane and trastuzumab-containing regimens were randomised to either lapatinib plus capecitabine or capecitabine alone. The addition of lapatinib prolonged TTP (HR = 0.57, 95% CI 0.43–0.77; P < 0.0001) leading to the approval of this drug for the second-line treatment of HER2-positive MBC. A trend, albeit not statistically significant, for improved overall survival was also observed (HR = 0.78, 95% CI 0.55–1.12; P = 0.177) [23]. Although novel compounds are being developed with activity in the third-line setting of HER2-positive MBC, no drug is yet approved.
The positive results of trastuzumab in HER2-positive MBC patients coupled with its favourable toxicity profile, has led many physicians to continue the target treatment at the moment of disease progression. The benefit of continuing trastuzumab beyond disease progression was evaluated in a phase III trial, where patients progressing during trastuzumab were randomised to capecitabine monotherapy or capecitabine plus trastuzumab [24]. The primary endpoint of the study was reached showing a benefit of PFS in favour of trastuzumab (5.6 vs 8.2 months; p = 0.0338). The maintenance therapy with trastuzumab also increased the response rate (27% vs 48%; p = 0.115), but only a trend for OS was demonstrated (HR = 0.76; p = 0.25), probably due to the low number of patients since the trial was closed prematurely due to slow accrual.
Another strategy for the first-line treatment of HER2-positive and ER-positive MBC is the dual blockade of HER2 and ER, which was evaluated in two phase III trials. Trastuzumab plus anastrozole compared with anastrozole alone in 207 HER2-positive, ER-positive MBC patients led to an increase in PFS (4.8 vs 2.4 months; p = 0.0016). No statistically significant overall survival gain was observed OS (28.5 vs 23.9 months; p = 0.325), however 70% of patients in the control arm received trastuzumab at progression [22, 25]. The combination lapatinib plus letrozole was compared to letrozole monotherapy in the first-line treatment of MBC. Median PFS in the HER2-positive and ER-positive subset increased from 3–8.2 months with combined therapy (HR = 0.71; 0.53–0.06; p = 0.019) [26].
One of the main concerns of anti-HER2 therapy is its associated cardiotoxicity. An appropriate patient selection before treatment initiation is mandatory and only patients with preserved cardiac function are deemed eligible for this class of treatment [27]. Diarrhoea is a common lapatinib-associated toxicity, and its management should be made in accordance with guidelines such as those provided by the American Society of Clinical Oncology [28].
Antiangiogenic therapy for metastatic breast cancer
Vascular endothelial growth factor (VEGF) is one of the most important pro-angiogenic factors involved in tumour growth [29, 30]. VEGF is usually secreted by cancer cells, tumour-associated stromal cells, and from various host cells, such as platelets and muscle cells [31]. The interaction of VEGF with other signalling pathways and growth factors mediates endothe-lial cell proliferation, survival, and vascular permeability.
Numerous agents that target the VEGF pathway are in clinical development, including agents targeting the VEGF ligand and agents targeting the VEGF receptors (VEGFRs). Bevacizumab is a humanised recombinant monoclonal antibody designed to block VEGFR-A. In a randomised clinical trial including patients with HER2-negative MBC, the addition of bevacizumab to paclitaxel increased response rate and doubled PFS (5.9 vs 11.8 months) but with no benefit in OS [32]. The addition of bevacizumab to other types of chemotherapy in first-line treatment of HER2-negative MBC, also showed benefits on PFS, but no benefits in survival [32–34]. Adding bevacizumab to chemotherapy increases the risk of hypertension, proteinuria, neurotoxicity, and left ventricular dysfunction, while highly increasing the cost of treatment.
Conclusion
Treatment of MBC remains challenging and international guidelines such as those of European School of Oncology–MBC Task Force are important for guiding treatment decisions. The importance of determining hormone and HER2 receptors is emphasised above, as the treatment modalities chosen are adapted in accordance with these tumour characteristics. A limiting factor however is the assessment of these receptors in the primary breast tumour. Recent studies have suggested that a significant proportion of relapsed lesions may have a change in the hormone and/or HER2 receptor status from the original tumour. The possibility of discordant characteristics between primary and metastatic tumour has motivated breast cancer treatment centres to indicate biopsy of metastatic sites whenever possible to guide treatment decisions according to the biology of metastatic disease [35].
Since MBC is still virtually incurable, issues of quality of life, symptom control and comfort take centre stage. For example, weekly therapeutic IV regimens can pose problems to patients living far from cancer centres and can usually be adapted on an individual basis without detrimental results.
It is of paramount importance to discuss early on with the patient the goals of treatment and the prognosis of the disease and to include the patient as much as possible in the treatment decision process, while respecting her/his will. It is also important to highlight the crucial role of a multidisciplinary team including medical, radiation, surgical, imaging, palliative care, and psycho-social specialists, for an accurate management of these patients. Since few standards of care exist for MBC and in view of the still dim prognosis of this disease, the possibility to participate in clinical trials should be offered to every patient whenever available.
Author for correspondence
Professor Fatima Cardoso, MD
Head, Breast Cancer Unit and Breast Cancer Research Director
Champalimaud Cancer Center
Av De Brasília – Doca de Pedrouços
PT-1400-048 Lisbon, Portugal
Co-authors
Otto Metzger Filho, MD
Research Fellow
Ivana Bozovic-Spasojevic, MSc, MD
Clinical and Translational Research Fellow
Breast International Group
Jules Bordet Institute
7/F, 121 Boulevard de Waterloo
BE-1000 Brussels, Belgium
References
1. Breast cancer facts and figures 2007–2008 and surveillance research 2007, American Cancer Society.
2. Bernard-Marty C, Cardoso F, Piccart MJ. Facts and controversies in systemic treatment of metastatic breast cancer. The Oncologist. 2004;9(6):617-32.
3. Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004 Jan 1; 100(1):44-52.
4. Greenberg PA, Hortobagyi GN, Smith TL, Ziegler LD, Frye DK, Buzdar AU. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996 Aug;14(8):2197-205.
5. Perez EA. Impact, mechanisms, and novel chemotherapy strategies for overcoming resistance to anthracyclines and taxanes in metastatic breast cancer. Breast Cancer Res Treat. 2009 Mar;114(2):195-201.
6. Porkka K, Blomqvist C, Rissanen P, Elomaa I, Pyrhonen S. Salvage therapies in women who fail to respond to first-line treatment with fluorouracil, epirubicin, and cyclophosphamide for advanced breast cancer. J Clin Oncol. 1994 Aug;12(8):1639-47.
7. Piccart-Gebhart MJ, Burzykowski T, Buyse M, Sledge G, Carmichael J, Luck HJ, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008 Apr 20;26(12):1980-6.
8. Blum JL, Jones SE, Buzdar AU, Lo Russo PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999 Feb;17(2):485-93.
9. Fumoleau P, Largillier R, Clippe C, Dieras V, Orfeuvre H, Lesimple T, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004 Mar;40(4):536-42.
10. Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001 Oct 1;92(7):1759-68.
11. Reichardt P, Von Minckwitz G, Thuss-Patience PC, Jonat W, Kolbl H, Janicke F, et al. Multicenter phase II study of oral capecitabine (Xeloda(“)) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003 Aug; 14(8):1227-33.
12. Wist EA, Sommer HH, Ostenstad B, Risberg T, Bremnes Y, Mjaaland I. Oral capecitabine in anthracycline- and taxane-pretreated advanced/metastatic breast cancer. Acta oncologica (Stockholm, Sweden). 2004;43(2):186-9.
13. Perez EA, Lerzo G, Pivot X, Thomas E, Vahdat L, Bosserman L, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007 Aug 10; 25(23):3407-14.
14. Twelves C, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet PJ, et al. A phase III study (EMBRACE) of eribulin mesylate versus treatment of physician’s choice in patients with locally recurrent or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010 Jun 20;28(18 Suppl):CRA1004.
15. Cardoso F, Bedard PL, Winer EP, Pagani O, Senkus-Konefka E, Fallowfield LJ, et al. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009 Sep 2;101(17):1174-81.
16. Muss HB, Case LD, Atkins JN, Bearden JD 3rd, Cooper MR, Cruz JM, et al. Tamoxifen versus high-dose oral medroxyprogesterone acetate as initial endocrine therapy for patients with metastatic breast cancer: a Piedmont Oncology Association study. J Clin Oncol. 1994 Aug;12(8):1630-8.
17. Klijn JG, Blamey RW, Boccardo F, Tominaga T, Duchateau L, Sylvester R. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol. 2001 Jan 15;19(2):343-53.
18. Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006 Sep 20;98(18):1285-91.
19. Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002 Aug 15;20(16):3396-403.
20. Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. Jama. 2009 Aug 19;302(7):774-80.
21. Bines J, Dienstmann R, Metzger O, Gonçalves AC, Claudino WM, Costa MF, et al. Prospective evaluation of megestrol acetate after aromatase inhibitor failure in advanced endocrine responsive breast cancer: Preliminary results. J Clin Oncol. 2010;28(Suppl 15):Abstract 1137.
22. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001 Mar 15;344(11):783-92.
23. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008 Dec;112(3):533-43.
24. von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009 Apr 20;27(12):1999-2006.
25. Mackey J, Kaufman B, Clemens M, et al. Trastuzumab prolongs progression-free survival in hormone-dependent and HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2006;100(Suppl 1):58;S3.
26. Johnston S, Pegram M, Press M, et al. Lapatinib combined with letrozole vs. letrozole alone for front line postmenopausal hormone receptor positive (HR+) metastatic breast cancer (MBC): first results from the EGF30008 Trial. San Antonio Breast Cancer Symposium General Section 4. 2008.
27. Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002 Oct 1;95(7):1592-600.
28. Benson AB 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, Jr., et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004 Jul 15;22(14):2918-26.
29. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Nat Acad Sci USA. 1998 Aug 4;95(16):9349-54.
30. Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008 Aug;8(8):579-91.
31. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008 May 8;358(19):2039-49.
32. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007 Dec 27;357(26):2666-76.
33. Miles DW, Chan A, Romieu G, Dirix LY, Cortés J, Pivot X, et al. Final overall survival (OS) results from the randomised, double-blind, placebo-controlled, phase III AVADO study of bevacizumab (BV) plus docetaxel (D) compared with placebo (PL) Plus D for the first-line treatment of locally recurrent (LR) or metastatic breast cancer (mBC). Cancer research. 2009;69(Suppl):24 495s.
34. Robert NJ, Dieras V, Glaspy J, Brufsky A, Bondarenko I, Lipatov O, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC). J Clin Oncol. 2009 May 20;27 (Suppl 15): Abstract 1005.
35. Simmons C, Miller N, Geddie W, Gianfelice D, Oldfield M, Dranitsaris G, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009 Sep;20(9):1499-504.