|
Abstract: |
Introduction
All medicinal products in the EU are subject to strict testing and assessment of their quality, efficacy, and safety before being authorised. Once placed on the market, they continue to be monitored to ensure that any aspect that could affect the safety profile of a medicine is detected and assessed, and that necessary measures are taken. This monitoring is called pharmacovigilance.
Good pharmacovigilance practice
Pharmacovigilance is the process and science of monitoring the safety of medicines and taking action to reduce the risks and increase the benefits of medicines. It is a key public health function [2].
The EU pharmacovigilance system
The EU pharmacovigilance system is now one of the most advanced and comprehensive systems in the world, and is a robust and transparent system that ensures a high level of public health protection throughout the EU.
The EU pharmacovigilance legislation was recently subject to a major review, and led to the implementation of new legislation in 2010. The new legislation, a ‘regulation’ and a ‘directive’ became applicable in July 2012.
Reasons for updating the system
The former EU pharmacovigilance legislation required updating to further strengthen pharmacovigilance. Statistics from EMA show that 5% of hospital admissions are a result of adverse drug reactions (ADRs); 5% of all hospital patients suffer an ADR; ADRs are the fifth most common cause of hospital death; an estimated 197,000 deaths per year in the EU are results from ADRs; and that the EU societal cost of ADRs are Euros 79 billion per year.
New legislation
The new legislation aims to promote and protect public health by reducing the burden of ADRs and optimising the use of medicines. This will be achieved through the delineation of clear roles and responsibilities; taking an evidence- and risk-based (proportionate) approach; increasing proactivity and planning; minimising duplication and redundancy; and integrating benefit and risk [1, 3].
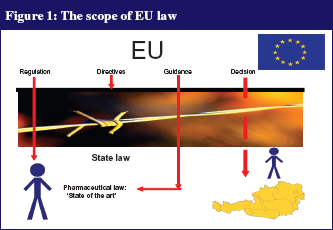
In order to understand the pharmacovigilance legislation, it is important to define some of the terms used by EMA, as subtle differences exist between the terms, see Table 1. The scope of EU law is shown in Figure 1.
In a descriptive analysis evaluating prescription drugs withdrawn from the worldwide market between 1960 and 1999 [6], 122 medications were withdrawn because of safety issues; 44.1% with European licence. The most common drugs were central nervous system acting substances (31.4%); non-steroidal anti-inflammatory drugs (13.2%); and antidepressant drugs (7.4%).
The most common problems associated with these drugs were hepatic (26.2%); haematological (10.5%); cardiovascular (8.7%); and carcinogenic (6.3%). Two-thirds of the medications had been marketed for at least 5.4 years, and one-third had been marketed for only two years [6].
In June 2012, EMA published its first set of guidelines on good pharmacovigilance practices. Seven out of 16 modules were finalised, each covering one major process in the safety monitoring of medicines [7]. These are the recent modules:
Module I : Pharmacovigilance systems and their quality systems
Module II : Pharmacovigilance systems master files
Module V : Risk management systems
Module VI : Management and reporting of adverse reactions to medicinal products
Module VII : Periodic safety update reports
Module VIII : Post-authorisation safety studies
Module IX : Signal management
Two further modules, i.e. Module III on ‘Pharmacovigilance inspections’ and Module X on processes for ‘Additional monitoring’ of medicinal products, were released for public consultation on 27 June 2012 and are envisaged to be finalized and published at the end of 2012 [8].
The remaining seven draft modules of the good pharmacovigilance practice package are under development, and were scheduled for an eight-week public consultation period during the third and fourth quarters of 2012, and will hopefully come into force at the beginning of 2013.
The new pharmacovigilance legislation also includes a modification to the definition of adverse reactions: a response to a medicinal product which is noxious and unintended. This is important for anyone working within the multi-professional team.
This includes adverse reactions arising from: (1) use of a medicinal product within the terms of the marketing authorisation; (2) use outside the terms of the marketing authorisation, including overdose, off-label use, misuse, abuse and medication errors; and (3) occupational exposure.
A periodic safety update report (PSUR) is intended to provide an update of the worldwide safety experience of a medicinal product to competent authorities at defined time points after authorisation. These reports are expected to summarise information succinctly, and evaluate the risk–benefit balance of the product critically in the light of new or changing information.
A course will be available to delegates with a working knowledge of the changing ICH (International Conference on Harmonisation) Guideline E2C and the new EU legislation for the purposes of planning, writing and reviewing periodic safety update reports. Group sessions and workshops are included to address practical issues and application of the regulations.
EMA has also established a new scientific committee entitled, the Pharmacovigilance Risk Assessment Committee (PRAC) [9]. It is responsible for assessing and monitoring all aspects of drug safety in those drugs that have been approved in the EU. Its role will specifically affect national authorisations. Recommendations by PRAC are considered by the Committee for Medicinal Products for Human Use (for centrally authorised products) and the Co-ordination Group for Mutual Recognition and Decentralised Procedures–Human (for all other drugs). Its role, however, is purely advisory, and a thorough, public justification must be given for implementing any recommendations made by PRAC.
PRAC also assumes responsibility for referral procedures, approving post-authorisation safety studies, and coordinating the review of periodic safety update reports, which will in future be provided only by EMA.
The new Directive contains a transitional period for the introduction of the pharmacovigilance system master file, which will be concluded in July 2015. It is mandatory for all new marketing authorisation applications submitted after 2 July 2012 for centrally authorised products, and 21 July 2012 for all other authorisation types. New marketing authorisation applications must be introduced on renewal of products in the transition period. By the end of the transition period, pharmacovigilance system master files will have to be in place for all products authorised in the EU and the European Economic Area.
Conclusion
The new good pharmacovigilance practice legislation aspires to excellent protection and promotion of public health [1]. It draws on all relevant data sources, uses health data and epidemiology to support the drug lifecycle, and is evidence-based.
The new legislation offers a rare opportunity to strengthen and rationalise public health, however, full and effective implementation will require a great deal of work [1, 10].
For our patients, collaboration within the EU is the key [1].
Author
Doris Haider, MBA, aHPh
Sozialmedizinisches Zentrum Süd
Kaiser-Franz-Josef-Spital
3 Kundratstrasse
AT-1100 Vienna, Austria
References
1. European Medicines Agency [homepage on the Internet]. The new pharmacovigilance legislation: an EMA perspective. 2012 [cited 2012 Dec 11]. Available from: www.ema.europa.eu/docs/en_GB/document_library/Presentation/2011/06/WC500107888.pdf
2. European Medicines Agency [homepage on the Internet]. Pharmacovigilance. 2012 [cited 2012 Dec 6]. Available from: www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000258.jsp&mid=WC0b01ac05800241de
3. GaBI Online – Generics and Biosimilars Initiative. Practical guidance on new pharmacovigilance legislation. [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Dec 11]. Available from: http://gabionline.net/Policies-Legislation/Practical-guidance-on-new-pharmacovigilance-legislation
4. EUR–LEX, European Law Database [homepage on the Internet]. 2012 [cited 2012 Dec 6]. Available from: http://eur-lex.europa.eu/en/index.htm
5. Craig P, de Búrca G. EU Law, Text, Cases and Materials. 4th ed. Oxford, New York: Oxford University Press; p. 86.
6. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets – 1960 to 1999. Drug Information Journal. 2001;35:293-317.
7. European Medicines Agency [homepage on the Internet]. European Medicines Agency finalises first set of guidelines on good pharmacovigilance practices. 2012 [cited 2012 Dec 11]. Available from: www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2012/06/news_detail_001546.jsp&mid=WC0b01ac058004d5c1
8. European Medicines Agency [homepage on the Internet] Guidelines on good pharmacovigilance practices: introductory cover note to finalisation of the first seven modules and public consultation of draft modules III and X. 2012 [cited 2012 Dec 11]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000345.jsp&mid=WC0b01ac058058f32c#section2
9. GaBI Online – Generics and Biosimilars Initiative. EMA a step closer to implementing new pharmacovigilance rules [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Dec 11]. Available from: www.gabionline.net/Policies-Legislation/EMA-a-step-closer-to-implementing-new-pharmacovigilance-rules
10. European Medicines Agency [homepage on the Internet]. Implementation of the new pharmacovigilance legislation. 2012 Implementation plan. 2012 [cited 2012 Dec 6]. Available from: www.ema.europa.eu/docs/en_GB/document_library/Presentation/2012/02/WC500123474.pdf