|
Study objectives: Protein-based medicines have specific storage requirements to ensure chemical stability and purity, but exposure to different environmental conditions can occur in normal use. The effect of extended unrefrigerated storage and thermal cycling on the stability of Hospira filgrastim (Nivestim) was examined. Methods: Three batches of two presentations of Hospira filgrastim prefilled syringes (30 MU and 48 MU) were kept refrigerated until 1–8 months after expiry. After this period, the samples were exposed to either three cycles of storage at 25 ± 2°C and 5 ± 3°C, seven days at 25 ± 2°C in light or dark conditions, three cycles at 25 ± 2°C and 5 ± 3°C, followed by seven days at room temperature (light and dark), or frozen for three days. Expired control samples were maintained at 5 ± 3°C. Samples were analysed for appearance, pH, particulate matter, protein concentration, impurities, biological activity and sterility. Results: All of the parameters measured for each sample of Hospira filgrastim were within the shelf life specification requirements, and there was no qualitative difference between parameters measured in samples that had undergone environmental stressing versus those maintained at 5 ± 3°C. Conclusion: Hospira filgrastim formulations are unaffected by cyclical changes in temperature between the fridge and 25 ± 2°C, and are also unaffected by exposure either to room temperature for seven days or to freezing for three days. Therefore, physicians, pharmacists and patients can be confident that Hospira filgrastim remains active and stable during environmental excursions commonly encountered in general use. |
Keywords: Filgrastim, G-CSF, refrigeration, stability, temperature
Study objectives and introduction
Neutropenia is a frequently occurring complication of myelosuppressive chemotherapy [1]. Neutropenia results in increased susceptibility to infection [1, 2] and can require treatment with anti-infectives. Patients with neutropenia are frequently admitted to hospital, and the condition can also require modification of chemotherapy regimens with the potential to compromise therapy outcomes [2]. The mainstay of therapy for neutropenia is recombinant granulocyte colony-stimulating factor (G-CSF, r-metHuG-CSF, rHuG-CSF, filgrastim) a cytokine that stimulates proliferation of haematopoietic progenitor cells that form mature neutrophils [3].
The manufacturing and formulation of biopharmaceuticals has inherent variability arising from differences in cellular expression systems and other manufacturing details. Recently, biosimilar filgrastims have become available. Recognising the variability in biopharmaceutical manufacture, EMA has issued guidance on standards that must be met for biosimilar filgrastims to obtain regulatory approval for medicinal use [4–7]. These standards are far more stringent than those required for approval of small molecule drugs, and include the requirement to conduct comprehensive assessments of quality, activity and clinical effectiveness [4–7]. In accordance with EMA requirements, the biosimilar Hospira filgrastim (Nivestim) has been studied in a development programme that included preclinical studies, two phase I clinical trials and one phase III clinical trial. The preclinical and phase I studies demonstrated the pharmacodynamic and pharmacokinetic equivalence of Hospira filgrastim versus its reference product Amgen filgrastim (Neupogen) [8–10].
The phase III study confirmed the bioequivalence of Hospira filgrastim and Amgen filgrastim in a randomised, multicentre trial of 279 patients undergoing myelosuppressive chemotherapy [11]. In the phase III study, Hospira filgrastim exhibited a manageable safety profile. The most common adverse event was bone pain, which is also the same for Amgen filgrastim [11].
As a result of these clinical trials, Hospira filgrastim gained approval for the treatment of neutropenia and the prevention of febrile neutropenia in cancer patients treated with cytotoxic chemotherapy (except those with myelodysplastic syndromes and chronic myeloid leukaemia) [12]. Hospira filgrastim is also indicated for the reduction in the duration of neutropenia in patients undergoing myeloablative therapy to support bone marrow transplantation, for the treatment of neutropenia in patients with human immunodeficiency virus, and for the treatment of severe congenital, cyclic or idiopathic neutropenia [12].
All protein-based biological medicines require careful handling and storage, to ensure chemical stability and to maintain a long shelf life. However, there is a need to understand what degree of flexibility exists regarding storage parameters, so that physicians can have the confidence to administer drugs that have been exposed to different environmental conditions, and so that healthcare teams can offer practical advice to patients on storage and handling. Therefore, a biochemical characterisation study was conducted to explore the effect of extended unrefrigerated storage, thermal cycling and freezing on the stability of Hospira filgrastim.
Methods
This study tested two formulations of Hospira filgrastim: the 30 MU (300 µg/0.5 mL) prefilled syringe, and the 48 MU (480 µg/0.5 mL) prefilled syringe. The third presentation of Hospira filgrastim is 12 MU (120 µg/0.2 mL), which is an under-filled version of the 30 MU presentation, and was not tested in these experiments.
Three batches of each of the two presentations were kept in refrigerated storage (2°C–8°C) for 31–38 months from manufacture (refrigerated shelf life 30 months). After this period, the samples were subjected to four types of environmental excursion:
- Cyclic test. Samples were stored for 2 days at 25 ± 2°C (hereafter referred to as 25°C), followed by 2 days storage at 5 ± 3°C (hereafter referred to as 5°C). This storage pattern was repeated three times (cycles) for each sample.
- Seven days at room temperature. Two sets of samples were exposed to seven days at room temperature (25°C), one set was exposed to light, and the other set was kept in the dark (wrapped in aluminium foil).
- Cyclic test, followed by seven days at room temperature. This test involved the cyclic test, followed by seven days exposure to room temperature (25°C), again with one set of samples exposed to light and the other set wrapped in foil.
- Freezing test. Samples were exposed to -20 ± 5°C (hereafter referred to as -20°C) for three days before returning to standard refrigerated storage.
Each sample was analysed for physicochemical characteristics, chemical purity and biological activity, see Table 1. Physical appearance was assessed visually. pH and particulate matter were measured in accordance with the European Pharmacopoeia 6.8 [13]. Protein concentration was measured spectroscopically using the method established by Groves et al. [14, 15].
Each sample was tested for protein impurities. The battery of tests used were designed to detect all protein species that differ from filgrastim, including misfolded, oxidised and dimeric forms, which are known to occur when filgrastim degrades. The quantity of all impurities in each sample was determined via sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), size-exclusion high-performance liquid chromatography (SEC-HPLC), isoelectric focussing (IEF), reverse phase HPLC (RP-HPLC) and ion chromatography (IC). For SDS-PAGE, samples were separated on a 13% polyacrylamide gel under reducing and non-reducing conditions according to methods set out in the European Pharmacopoeia 6.8 [13]. The required standard for SDS-PAGE was that the principal silver-stained band in the electropherogram obtained with the test solution should be similar in position to the principal band obtained with the reference solution, Filgrastim Reference Substance 1920, which is an in-house standard calibrated against the international standard of G-CSF 88/502. Molecular weights should be between 14–21 kDa using standards from GE Healthcare, or between 14.4–21.5 kDa using standards from Bio-Rad.
An IEF system (GE Healthcare) was used to determine the isoelectric point for each sample, and for detection of impurities with isoelectric points differing from that of the reference filgrastim. Samples were separated on an IEF gel containing 6% acrylamide and 3 M urea. The pH gradient was 4.0–8.0, and proteins were visualised with Coomassie Brilliant Blue R-250 [13]. The required standard for IEF was that the principal band in the electropherogram obtained with the test solution should be in a similar position to the principal band in the electropherogram obtained with the reference solution.
The SEC-HPLC method was used to determine impurities with molecular masses higher than that of filgrastim, and was based on the method published in the European Pharmacopoeia 6.8 [13]. SEC-HPLC was performed using an Agilent HPLC machine equipped with a diode array. Separation was achieved using a silica-based column from Tosoh Bioscience (TSKgel G3000SWXL; 7.8 × 300 mm; 5 µm); using a flow rate of 0.5 mL/min. Lower limit of quantitation (LLOQ) for SEC-HPLC was 0.20%.
The quality of the filgrastim assay and degradation products in each sample was determined via RP-HPLC using the method published in the European Pharmacopoeia 6.8 [13]. Reverse phase high-performance liquid chromatography analyses were performed on the same machine used for SEC-HPLC, but with a Phenomenex Jupiter C4 column (4.6 × 250 mm; 5 µm) with a linear 60 min gradient of acetonitrile in 0.1% trifluoroacetic acid, and a flow rate of 0.6 mL/min.
f-met filgrastim, an established degradation product of filgrastim, and impurities with greater acidity than filgrastim were determined via IC using an in-house method. Analyses were performed on an Agilent fluorescence HPLC, and separation was achieved using a Tosoh Bioscience TSKgel SP-5PW column (7.5 mm × 75 mm; 10 µm) with a methacrylic polymer stationary phase, an n-propyl-sulphonate functional group and a 20 min linear gradient. The mobile phases were sodium acetate trihydrate 0.02 mol/L (pH = 5.4) and sodium chloride 0.5 mol/L, with a flow rate of 1.0 mL/min.
Potency was assessed according to the European Pharmacopoeia standards [13] via a validated biological assay in M-NFS-60 murine myeloblastic cells. The required standard was 0.9 × 108–1.5 × 108 IU/mg total protein in the sample. Sterility was assessed using a membrane filtration method. Concentrations of bacterial endotoxins should remain < 40 IU/mg.
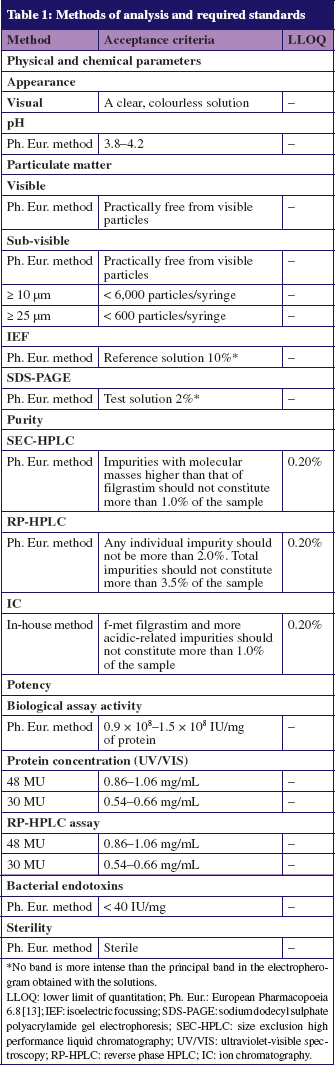
All methods used for product quality control were validated in accordance with International Conference on Harmonisation guidelines. Analysis methods, required standards and LLOQ are shown in Table 1.
Results
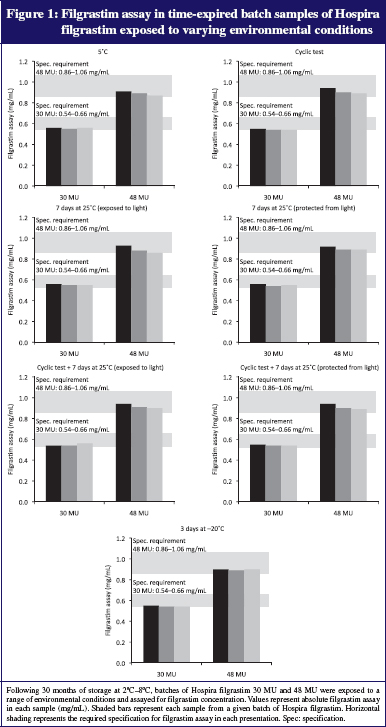
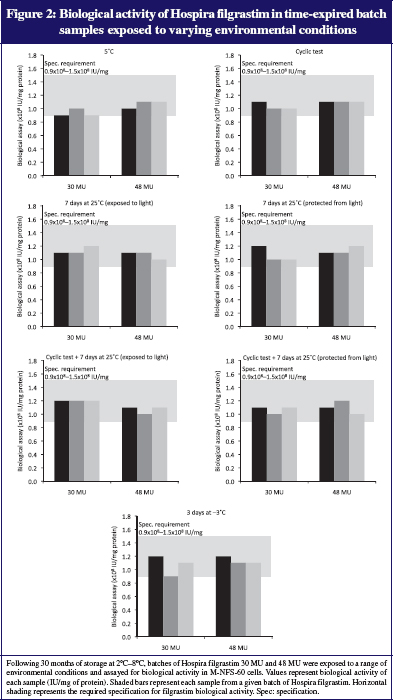
There were no significant differences in filgrastim concentrations between samples undergoing environmental excursions with those maintained at 5°C. Protein concentrations and filgrastim assay remained within specified limits derived from requirements of drug regulatory authorities (filgrastim assay limits: 0.54–0.66 mg/mL for 30 MU samples and 0.86–1.06 mg/mL for 48 MU samples; see Figure 1). Biological potency was also maintained in all samples, and remained within the pre-specified limits of 0.9 × 108–1.5 × 108 IU/mg of protein, see Figure 2.
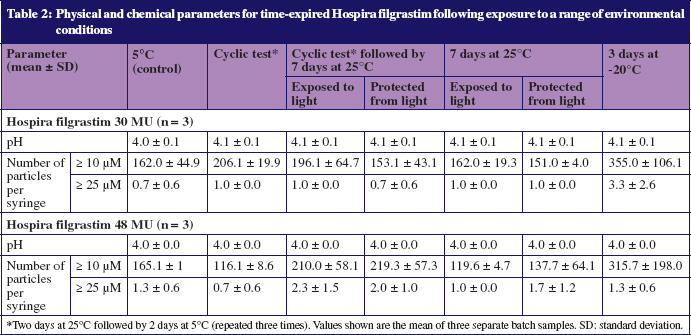
All samples were clear and colourless, in-line with the required specifications of Hospira filgrastim, and the pH of all samples was in the range 4.0–4.1, also in line with the required specification, see Table 1. No particles were visible in any tested samples, and all results complied with requirements for sub-visible particulate matter (≤ 6,000 particles ≥ 10 µM and ≤ 600 particles ≥ 25 µM per syringe, see Table 1).
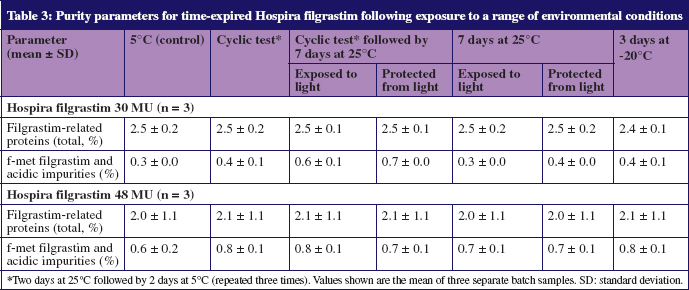
Data from SDS-PAGE, IEF, RP-HPLC, IC and SEC-HPLC showed no evidence of impurities beyond the required ranges, see Table 2. The quantities of f-met filgrastim impurities and impurities of greater acidity than filgrastim, were slightly higher in 30 MU samples stored for seven days at room temperature following thermal cycling, than in samples stored continuously at 5°C; however, all concentrations remained below the 1.0% specification limit, see Table 3.
Bacterial endotoxin levels remained below 40 IU/mg (specific limit for Hospira filgrastim limit based on total daily dose in patients) in all samples and no microorganism contamination was evident following environmental variations.
Discussion
The therapeutic efficacy of protein-based medicines is dependent on the conformational structure of the protein molecule. However, proteins are complex, flexible structures and are sensitive to external environmental conditions [16]. Degradation of concentrated proteins in storage is generally caused by inter- and intra-molecular reactions such as hydrolysis, deamination, oxidation, aspartate isomerisation and aggregation [17]. Aggregation is the principal means by which all high-concentration proteins degrade, and can occur in response to changes in thermal conditions that affect conformational structure [17]. Various methods are now routinely used to combat aggregation. Lyophilisation is used to restrict protein mobility—lyoprotectants can also be added [17], and there is evidence that the specific molar ratios of stabilisers is important for successful lyophilisation [18]. For aqueous protein solutions, osmolytes, e.g. sugars, can be introduced to the formulation; these are excluded from the immediate protein microenvironment and are preferentially hydrated [17].
The chemical characterisation and formulation of filgrastim continues to be a topic of research to better understand the chemistry and stability of the product, and to ensure a safe and efficacious molecule [19]. Ultra-high throughput computational screening methods have been used to identify large numbers of G-CSF variants with different stability profiles. Using these methods, Luo et al. screened 1021–1028 G-CSF sequences that were based on alterations to 25–34 residues deep in the protein filgrastim core [20]. Optimal core designs were selected for biochemical and pharmacokinetic characterisation and efficacy testing. This process was able to identify filgrastim variants with long-term stability at temperatures as high as 13°C with 5–10-fold improvements in shelf life without the loss of biological activity [20].
Recent research efforts, such as high throughput screening, have yet to result in the generation of a G-CSF with improved storage requirements; however, the inherent stringency of EMA requirements for the development of European biosimilar G-CSFs [4–7], means that the required standards for the quality of the molecular and formulated products are the same as that of the originator. The guidelines issued by EMA that govern the requirements must be met for approval if biosimilars differ from the requirements set out for small molecule therapeutics. For small molecular weight drugs, e.g. chemotherapeutics and other non-protein drugs, demonstration of pharmacokinetic similarity is sufficient to gain approval; however, EMA recognises that the complexity of the manufacturing processes for protein therapeutics results in inherent variability in the final synthesised product. Therefore, therapeutic equivalence is difficult to determine without full-scale clinical trials [21]. With these issues in mind, EMA has issued strict guidance on the standards that must be met by original protein therapeutics and their biosimilar counterparts to obtain approval for therapeutic use in humans [4–7]. For follow-on biologics, these standards must be met in order for the drugs to be termed ‘biosimilar’. The EMA guidelines ensure therapeutic efficacy, protect patient safety and minimise the effects of inter-batch variability. The guidelines include the consideration of shelf life of the reference product and provide recommendations on the standards that must be met by any analytical techniques used [5].
The data presented in this study have demonstrated that Hospira filgrastim, a biosimilar G-CSF, can be exposed to cyclical changes in temperature between 5°C and 25°C (mimicking temperature excursions during transport and handling), exposure to 25°C for seven days or frozen for three days. All of the tests were done after the nominal expiry date of the product (30 months). Notably, no increases in the quantity of impurities with molecular weights higher than filgrastim were observed, thus demonstrating that none of the environmental alterations employed, resulted in aggregation. On the basis of these findings, the SmPC for Hospira filgrastim has been updated to inform pharmacists and physicians that they should not be concerned about exposure of Hospira filgrastim to room temperature conditions for a single period of seven days. While the drug can be used at any point during this time, it should be discarded at the end of the seven days. Although we did not test the 12 MU formulation of Hospira filgrastim, this is a smaller presentation of the same master batch as the 30 MU presentation; therefore, data collected on the 30 MU syringe can be extrapolated to the 12 MU syringe. Hospira continues to test formulations of its filgrastim presentations, to further define their stability characteristics.
The data presented here, and the update of the SmPC for Hospira filgrastim that was based on them, provide genuine practical benefits for patients receiving G-CSF, to support myelosuppressive chemotherapy. For instance, many courses of G-CSF therapy are 3–5 days in duration [22], commencing 24–72 hours after the end of the chemotherapy cycle [23, 24]. Therefore, under the updated storage instructions for Hospira filgrastim, the drug does not require refrigerated storage after dispensing from the hospital pharmacy at the time of patient discharge, as long as the drug is used within seven days. This may be beneficial to patients who lack reliable refrigerated storage space, or those who are planning extended periods away from home; however, refrigeration should be used for G-CSF courses lasting for six days or more, or for regimens that require commencement of G-CSF therapy more than 24 hours after dispensing of Hospira filgrastim in the cycle [25].
In addition to obtaining data to support 7-day, out-of-fridge stability for Hospira filgrastim, the present study also gathered data demonstrating Hospira filgrastim is unlikely to be affected by accidental freezing or thermal cycling between 5°C and 25°C. The environmental conditions to which Hospira filgrastim was exposed to in the present study, reflect many of the genuine thermal variations that drugs can undergo in storage and transport, and the resulting data provide much needed confidence that frequently encountered changes in environmental conditions do not affect the stability or biological activity of Hospira filgrastim. These data are significant in the field, as they represent a step towards stability data gathered with the aim of improving patient compliance and ease of use, rather than investigating stability from a purely technical or commercial standpoint.
The 7-day, out-of-fridge stability at room temperature, is an important addition to the existing user-centred features for Hospira filgrastim, that include prefilled syringes with integrated needle-safe devices, a wide-range of presentations, the use of colour-highlighted doses on the packaging and no requirement for reconstitution. Recently, an additional filgrastim product has received approval for 72 hours out-of-fridge storage [26] which, while further highlighting the user requirements for additional stability information to support real-life handling and use of these drugs, falls short of providing out-of fridge coverage for routine use in 7-day regimens, as previously described. Additionally, Hospira filgrastim is the only G-CSF currently available that has a low-dose (12 MU) version in a prefilled syringe, thereby minimising wastage for patients who require low doses of filgrastim, and enabling a high degree of flexibility in designing treatment regimens. Therefore, among the currently available G-CSFs, Hospira filgrastim provides a range of features that provide direct benefits to pharmacists and patients in a product that continues to be developed with the user in mind. The clinical community looks forward to additional technical innovations and molecular characterisation studies for G-CSFs that may further improve the convenience of these agents for patients.
Conclusion
In conclusion, current Hospira filgrastim formulations are unaffected by cyclical changes in temperature between the fridge and room temperature (not above 25°C), and are also unaffected by exposure to room temperature (not above 25°C) for seven days or frozen for three days. After exposure to 25°C for seven days, Hospira filgrastim should not be returned to the fridge. These findings result in benefits of convenience and confidence for physicians, pharmacists and patients concerned with the supply, storage and administration of G-CSF, and highlight how the seemingly technical exercise of exploring the long-term thermal stability of a growth factor therapeutic can lead to tangible benefits for prescribers, pharmacists and patients. Future investigations of protein therapeutics should include stability studies of the type presented here, to ensure that the drugs made available to patients meet the current demands of storage, handling and flexibility.
Acknowledgements
All of the analyses were conducted by Hospira Zagreb doo Quality Department, with the exception of sterility and bacterial endotoxins tests, which were conducted by Pliva Hrvatska doo. Data interpretation was provided by the authors. The authors would like to thank Dr Nigel C Eastmond of Eastmond Medicomm Ltd who provided editorial support, which was funded by Hospira Inc.
Conflict of interest
Mr Bruce Burnett has received financial support as a consultant to Hospira, Teva and Ratiopharm. Ivona Radić Krleža is an employee of Hospira Zagreb doo.
Author for correspondence
Bruce Burnett, MMedSci (Clinical Oncology)
North Wales Cancer Treatment Centre
Glan Clwyd Hospital
Rhyl, Denbighshire LL18 5UJ, UK
Co-author
Ivona Radić Krleža, BSc (Pharm Tech)
References
1. Dale DC. Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs. 2002;62 Suppl 1:1-15.
2. Lyman GH. Risks and consequences of chemotherapy-induced neutropenia. Clin Cornerstone. 2006;8 Suppl 5:S12-8.
3. Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth factors. 2005;23(1):33-41.
4. European Medicines Agency Committee for Medicinal Products for Human Use. Annex to guidelines on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Guidance on similar medicinal products containing recombinant granulocyte-colony stimulating factor. 2006 [cited 2012 Jun 10]. Available from: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003955.pdf
5. European Medicines Agency Committee for Medicinal Products for Human Use. Guidelines on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues. 2006 [cited 2012 Jun 10]. Available from: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003953.pdf
6. European Medicines Agency Committee for Medicinal Products for Human Use. Guidelines on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. 2006 [cited 2012 Jun 10]. Available from: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf
7. European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on similar biological medicinal products. 2005 [cited 2012 Jun 10]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf
8. Skrlin A, Radic I, Vuletic M, Schwinke D, Runac D, Kusalic T, et al. Comparison of the physicochemical properties of a biosimilar filgrastim with those of reference filgrastim. Biologicals. 2010;38(5):557-66.
9. Waller CF, Bronchud M, Mair S, Challand R. Comparison of the pharmacodynamic profiles of a biosimilar filgrastim and Amgen filgrastim: results from a randomized, phase I trial. Ann Hematol. 2010;89(10):971-8.
10. Waller CF, Bronchud M, Mair S, Challand R. Pharmacokinetic profiles of a biosimilar filgrastim and Amgen filgrastim: results from a randomized, phase I trial. Ann Hematol. 2010;89(9):927-33.
11. Waller CF, Semiglazov VF, Tjulandin S, Bentsion D, Chan S, Challand R. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 2010;33(10):504-11.
12. Hospira Inc. Nivestim summary of product characteristics. 2010 [cited 2012 Jun 10]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001142/WC500093661.pdf
13. European Directorate for the Quality of Medicines. Filgrastim concentrated solution (2206). European Pharmacopoeia. 2010;6(8):5931-3.
14. Groves WE, Davis FC, Jr., Sells BH. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968;22(2):195-210.
15. Stoscheck CM. Quantitation of protein. In: Deutcher MP, editor. Methods in enzymology. San Diego: Academic Press; 1990. p 50-68.
16. Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4(4):298-306.
17. Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93(6):1390-402.
18. Cleland JL, Lam X, Kendrick B, Yang J, Yang TH, Overcashier D, et al. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J Pharm Sci. 2001;90(3):310-21.
19. Herman A, Boone T, Hseing L. Characterization, formulation, and stability of Neupogen® (filgrastim), a recombinant human granulocyte-colony stimulating factor. Pharm Biotechnol. 2002;9:303-28.
20. Luo P, Hayes RJ, Chan C, Stark DM, Hwang MY, Jacinto JM, et al. Development of a cytokine analog with enhanced stability using computational ultrahigh throughput screening. Protein Sci. 2002;11(5):1218-26.
21. Schellekens H. Biosimilar therapeutics-what do we need to consider? NDT Plus. 2009;2(Suppl 1):i27-i36.
22. National Health Service. Guidelines for the use of G-CSF following chemotherapy. 2010 [cited 2012 Jun 10]. Available from: www.swshcn.nhs.uk/healthcare-professionals/clinical-policies-and-protocols/supportive-care/g-csf_network_policy_v1_7.07.pdf
23. Crawford J, Caserta C, Roila F. Hematopoietic growth factors: ESMO recommendations for the applications. Ann Oncol. 2010;21 Suppl 5:v248-51.
24. Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187-205.
25. Scott SD, Chrischilles EA, Link BK, Delgado DJ, Fridman M, Stolshek BS. Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin’s lymphoma treated with chemotherapy. J Manag Care Pharm. 2003;9(2 Suppl):15-21.
26. Sandoz. Zarzio summary of product characteristics. 2010 [cited 2012 Jun 10]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000917/WC500046525.pdf