
Last update: 11 June 2013
Confusion may sometimes surround terms used in the fields of generics and biosimilars. This has been recognised as a problem by EMA, who has expressed the need to propose a more precise definition for biosimilars due to problems arising from imprecise usage of the terms in the scientific literature and elsewhere.
The source of some of this confusion is due to authorities in various regions of the world defining terms differently and other instances are due to a misunderstanding of the actual nature, characteristics, and method of research and manufacture of these biological products.
In an attempt to prevent any such confusion for GaBI Online readers, here is a glossary of the relevant terms for biosimilars and generics as used in GaBI Online.
Active substance
Active ingredient or molecule that goes into a specific medicine and provides this medicine with properties for treating or preventing one or several specific disease(s).
Adverse event
The occurrence of an undesirable, unpleasant or life-threatening reaction to a medicinal product.
Adverse reaction
A response to a medicinal product, which is noxious and unintended.
Amino acid
Building blocks of proteins. There are 20 common amino acids found in proteins.
Anaemia
A condition that is due to a reduced number of red blood cells or reduced amounts of haemoglobin within them. This results in reduced oxygen-carrying capacity and reduced aerobic activity in blood cells. The symptoms include marked tiredness. Anaemia often occurs after chemotherapy.
Anaphylaxis
An acute and severe allergic reaction in humans.
Antibody (pl: antibodies)
Antibodies (also known as immunoglobulins, abbreviated to Ig) are proteins found in blood or other bodily fluids. They are produced by humans and animals in response to the presence of a specific antigen such as micro-organism, e.g. virus, bacterium. They are used by the immune system to identify and neutralise foreign objects, such as bacteria and viruses by binding to an antigen in a manner that leads to immunological responses such as death of bacteria, elimination of foreign proteins, etc. Usually antibodies are very useful and allow individuals to deal with current and subsequent infections. They can become a problem in some circumstances, e.g. autoimmune disease.
Antigen
A substance, often a protein, which stimulates the production of antibodies. Examples can include pollen grains, dust, bacteria or viruses.
Aplasia
Refers to a type of anaemia affecting the precursors to red blood cells but not to white blood cells.
Assay
Technique for measuring a biological response.
Autoimmune disease
A disease caused by the body producing an excessive immune response against its own tissues. Thereby, the immune system ceases to recognise one or more of the body’s normal constituents as ‘self’ and will create auto-antibodies that attack its own cells, tissues, and/or organs. Inflammation and tissue damage are common symptoms of autoimmune diseases.
Autoimmunity
A condition in which the body mounts an immune response against one of its own organs or tissues.
Automatic substitution
The practice by which a product other than the one specified on the prescriptions is dispensed to the patient, without the prior informed consent of the treating physician. A variation of substitution is practised in some countries, where, if the physician prescribes by International Nonproprietary Name (INN), the pharmacist may decide to dispense any product with the same active ingredient.
Bacteria
Very small, simple living organisms. Their living processes can be ‘hijacked’ by genetic engineering technology and used to manufacture biotechnology medicines.
Bioassay
Determination of the effectiveness of a compound by measuring its effect on animals, tissues or organisms in comparison with a standard preparation. Bioassays using animals or organs are now rarely used to measure potency of biologicals.
Bioequivalence
The absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of action when administered at the same molar dose under similar conditions in an appropriately designed study.
Biological medicinal product
EMA Definition
A biological medicinal product is a medicinal product whose active substance is made by or derived from a living organism.
Other Explanation
A medicinal product or a vaccine that consists of, or has been produced by the use of, living organisms. Often recombinant DNA (a form of DNA that does not exist naturally and which combines DNA sequences that would not normally occur together in order to establish new functions) forms the basis for biotechnologically manufactured products. Examples include therapeutic proteins such as antibodies, insulin or interleukins; but also vaccines, nucleic acids or tissues and cells.
Biologicals have large, complex, inherently diverse molecular structures that are not easily identified or characterized, and many are manufactured using biotechnology. Biological products often represent the cutting edge of biomedical research and are sometimes the most effective way to prevent or treat a disease.
Biotechnology medicines are derived through three main sources: yeast cells, bacterial cells and mammalian cells. These host cells are genetically modified and are allowed to multiply in a process referred to as fermentation. Human protein, i.e. the active ingredient of the future medicine, is either produced and contained within the host cells, or secreted into a nutrient solution. The cells and their human protein products are then removed, separated, purified and processed into a formulated medicine.
Biopharmaceuticals
Medicines made, or derived, from living organisms using biotechnology. See also biological medicine.
Biosimilar medicinal product
EMA Definition
A biosimilar medicinal product is a medicinal product, which is similar to a biological medicinal product that has already been authorised (the ‘biological reference medicinal product’). The active substance of a biosimilar medicinal product is similar to the one of the biological reference medicinal product.
The name, appearance and packaging of a biosimilar medicinal product may differ to those of the biological reference medicinal product. It may also contain different inactive ingredients.
Other Explanation
Medicine that is approved by the regulatory authorities as being similar in terms of quality, efficacy and safety to a biological reference medicinal product with which it has been compared. Biosimilar medicines are intended to be close molecular copies of the originator’s product. However, they may not be identical copies. They do, however, depend on the same mechanism of action and are intended to be used for the same therapeutic indication. A similar, but not identical, version of an existing biological medicine made following the patent expiry of the original product (must be made by a different manufacturer).
Biosimilarity
Property of a medicine to show similarity and lack of significant differences in terms of quality, efficacy and safety to a reference biological medicine to which it has been compared.
Biotechnology medicine
See biological medicinal product.
Biotechnology
A broad term generally used to describe the use of biology in industrial processes such as agriculture, brewing and baking. Recently, the word has come to refer more to the production of genetically modified organisms or the manufacture of products, notably medicines, from genetically modified organisms. Technology that manipulates living organisms so that they produce certain specific proteins including hormones or monoclonal antibodies; or any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use.
Cell culture
The process by which cells may be grown outside the body under controlled conditions.
Characterisation
Tests to determine the properties of a molecule or active substance, e.g. molecular size/weight, chemical structure, purity. These tests are also called physiochemical characterisation.
Chemotherapy
The application of chemicals (drugs) to control the growth of cells that form a cancer.
Clinical study or trial
Study with the objective of determining how a (new) medicine is handled by, and affects, humans. Clinical studies or trials are conducted in healthy volunteers or in patients. Clinical studies routinely involve the use of a control group of patients that is given an inactive substance (placebo) that looks like the test product. Pivotal clinical studies involving a larger group of patients provide evidence on whether the medicine can be considered both safe and effective in a real clinical setting. There are four types of clinical studies:
- Phase I clinical study or trial
Study with the objectives of determining how a medicine is handled by, and affects, humans, and of helping to predict the initial dosage range for the medicine and to test the safety profile. Although such studies are often conducted in a small cohort of healthy volunteers, phase I studies in patients are also possible in some situations. - Phase II clinical study or trial
Study of a small number of patients with the objectives of proving the efficacy concept of a medicine and of collecting data to establish the correct dose for that medicine. Phase II studies are not formally required for the development of biosimilar medicines as efficacy and dose are already established for the reference product. - Phase III clinical study or trial
Study involving a larger group of patients, which aims to provide definitive evidence on whether the medicine can be considered both safe and effective in a real clinical setting for drug registration. - Phase IV clinical study or trial
Study with the objective of collecting post-marketing surveillance data to track safety over time.
Comparability
The scientific evaluation of a comparison of two medicinal products to determine equivalence and any detectable differences at the level of quality, efficacy and safety.
Compulsory licence
A compulsory licence allows for a generic drug to be brought onto the market while the originator is still under patent protection under certain humanitarian conditions. For example, the 2005 Indian Patents Act allows for issuing of a compulsory licence in cases including those where:
a) the reasonable requirements of the public with respect to the patented invention have not been satisfied or;
b) it is not available to the public at a reasonably affordable price and;
c) the patent is not being worked.
The idea behind compulsory licensing is to provide low cost medicines and increase access to medicines for poorer people in a public health crisis.
Compound annual growth rate (CAGR)
The compound annual growth rate (CAGR) is the year-over-year growth rate of an investment over a specified period of time.
The CAGR is calculated by taking the nth root of the total percentage growth rate, where n is the number of years in the period being considered.
This can be written as follows: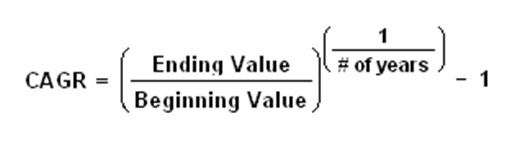
Defined Daily Doses (DDD)
The defined daily dose (DDD) is a statistical measure of drug consumption, defined by the World Health Organization (WHO). It is used to standardise the comparison of drug usage between different drugs or between different healthcare environments. The DDD is not to be confused with the therapeutic dose or with the dose actually prescribed by a physician for an individual patient.
The WHO’s definition is: ‘The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults.’
Diabetes
There are several forms of diabetes. It is a common condition in which the amount of glucose (sugar) in the blood is too high because the body is unable to use it properly. Normally, a hormone called insulin removes excess glucose from the blood.
DNA
Acronym for deoxyribonucleic acid. A molecule of DNA consists of a long chain of deoxyribose, a 5-carbon sugar, and phosphate groups with the bases adenine, thymine, cytosine and guanine. DNA contains the genetic code that controls the production of proteins in living things.
EMA
The European Medicines Agency, responsible for approving all medicines before they are made available to doctors and patients in the 27 Member States of the European Union.
Enzyme
A protein catalyst that facilitates specific chemical or metabolic reactions necessary for cell growth and reproduction.
Erythropoietin (epoetin – EPO)
A hormone released from the kidneys and the liver in response to low oxygen concentrations in the blood. It controls the rate of red blood cell production.
EudraVigilance
Data processing network and management system for reporting and evaluating suspected adverse reactions during the development and following the marketing authorisation of medicinal products in the European Economic Area (EEA).
Extrapolation
Extending the findings from one set of conditions to another, such as extending and applying the data from clinical studies regarding one medical condition to another medical condition or extending data from clinical studies in adults to children.
FDA
The US Food and Drug Administration. This agency is responsible for approving all medicines before they are made available to doctors and patients in the United States.
Fermentation
Chemical reactions induced by living organisms (or enzymes derived from living organisms) to produce raw material for pharmaceutical products.
Formulation
The recipe and presentation of a medicine.
Generic medicinal product
EMA Definition
A medicinal product which has the same qualitative and quantitative composition in active substances and the same pharmaceutical form as the reference medicinal product, and whose bioequivalence with the reference medicinal product has been demonstrated by appropriate bioavailability studies. (Reg. 726/2004, Art 10, 2b) Generic ‘copies’ can only be marketed after the originator’s patent protection and/or marketing exclusivity has expired.
Other Explanation
Medicine that has the same composition in active substance(s) and the same pharmaceutical form as the originator reference medicine, and whose bioequivalence with the originator reference medicine, i.e. the same behaviour in the body, has been demonstrated by appropriate bioequivalence studies. A generic medicine may be made by a different company after patent expiry of the originator product.
Genetic disorder
A condition that results from a defective gene or chromosome.
Genetic engineering
A technology used to alter the genetic material of living cells in order to make them capable of producing new substances or performing new functions.
Glycosylation
The type and length of any sugar or carbohydrate groups attached to a given molecule.
Haemoglobin
The protein found in the blood of most vertebrates and some invertebrates that carries oxygen and small amounts of carbon dioxide.
Harvesting
Separation of raw biological material from cell culture.
Identification
The action of designating or identifying something.
Immune response
The immune response is the way the body recognises and defends itself by producing antibodies against micro-organisms, viruses and substances recognised as foreign and potentially harmful to the body.
Immune system
The collection of mechanisms – cells, biological substances such as antibodies and cellular activities – within the body that work together to provide resistance to disease by identifying and killing pathogens, e.g. viruses and bacteria, and tumour cells.
Immunogenic/Immunogenicity
The capability of a specific substance to cause an immune reaction, i.e. induce the production of antibodies in the human body. The biological response to such a substance is termed an immune response or reaction.
In vitro and In vivo
Biological or chemical work done in the test tube (in vitro is Latin for ‘in glass’) rather than in living systems.
INN (International Nonproprietary Name)
EMA Definition
The INN identifies pharmaceutical substances or active pharmaceutical ingredients. Each INN is a unique name that is globally recognised and is public property. A nonproprietary name is also known as a generic name.
Other Explanation
Scientific or generic name of an active substance. The World Health Organization (WHO) in Geneva allocates INNs for new active substances. The INN is a unique and universally accessible name used to identify each pharmaceutical substance or active pharmaceutical ingredient. For generic and biosimilar medicines cross-referring to originator products, it is the regulatory authority that decides whether the INN of the active substance as submitted for the generic or the biosimilar medicine is scientifically acceptable.
Insulin
A hormone of vertebrates and invertebrates that promotes the conversion of glucose to glycogen.
Interchangeability
Refers to the medical/pharmaceutical practice of switching one medicine for another that is equivalent, in a given clinical setting. A product is considered to be interchangeable if it can be administered or dispensed instead of another clinically equivalence product without significant risk of an adverse health outcome.
Interferon
A class of proteins important in the immune response. There are three major types of interferon: alpha (leukocyte), beta (fibroblast) and gamma (immune). Interferon inhibits viral infections and may have anti-cancer properties.
Line extension
A variation of an existing product. The variation can be a new formulation of an existing product or a new modification of an existing molecular entity. Line extensions are used by companies to extend patent protection of a brand-name drug.
Marketing authorisation
The permission granted by a regulatory authority to a company to market a medicinal product in accordance with the conditions described on the label, following the company’s submission of required documentation and data relating to testing and clinical trials of the product.
Molecular
Of a molecule.
Molecule
Compound made by atoms in a fixed and specific arrangement held together by strong chemical bonds. Molecules are made up of one or more atoms. If they contain more than one atom, the atoms can be the same (an oxygen molecule has two oxygen atoms) or different (a water molecule has two hydrogen atoms and one oxygen atom). Biological molecules, such as proteins, can be made up of many thousands of atoms.
Monoclonal antibodies
Monospecific antibodies that are produced by a single clone of immune cells. They have become an important tool in molecular biology and medicine, and the basis of many biopharmaceuticals.
Neurodegenerative
A term used to describe diseases such as Parkinson’s disease and Alzheimer’s disease, which cause elements of the nervous system to deteriorate.
Nucleic acid
A macromolecule, i.e. a very large molecule, composed of chains of monomeric (having a single component) nucleotides, which are molecules that, when joined together, make up the structural units of RNA and DNA. In biochemistry, these molecules carry genetic information or form structures within cells.
Organism
A living thing. The term includes anything that has DNA, from bacteria to vertebrates.
Originator company
Company that was first to develop and produce a specific medicine (biological or pharmaceutical).
Originator medicinal product
Medicine that has been developed and produced by an originator company and that has been approved by the national regulatory authorities or the European Commission on the basis of a full registration dossier.
Patent
A patent is a legal mechanism granted by a State (national government) which allows the discoverer of a medicine the exclusive right to make and sell the product for a set period of time in order to recover the development costs, in exchange for public disclosure of their invention. Typically, however, a patent application must include one or more claims defining the invention, which must be new, non-obvious, and useful or industrially applicable.
Pharmaceuticals
Conventional or traditional chemical medicines.
Pharmacodynamic tests or studies
Study of the actions and effects of a medicine on living systems over a period of time.
Pharmacovigilance
Science and activities relating to the detection, assessment, understanding and prevention of any adverse effects of medicinal products placed on the market. These are safety control procedures to which medicines are subject before, during and after their approval by regulatory authorities.
Pharmcokinetic tests or studies
Studies to determine how medicines are absorbed, distributed metabolised and eliminated by the body.
Physiochemical characterisation
Tests to determine the properties of a molecule or active substance, e.g. molecular size/weight, chemical structure, purity.
Polypeptides
Molecules made up of chains of amino acids, which may be pharmacologically active in the human body. They contain fewer amino acids, and hence have lower molecular weights than proteins.
Post-Authorisation Safety Study
Any study with an authorised medicinal product conducted with the aim of identifying, characterising or quantifying a safety hazard, confirming the safety profile of the medicinal product, or of measuring the effectiveness of risk management measures after their approval by regulatory authorities.
Proteins
Large molecules made of amino acids arranged in chains, e.g. erythropoietin.
Purification
Processes used to remove impurities (foreign or undesired materials) from a medicinal product.
Reference product
The original product to which a biosimilar or generic drug refers in its application for marketing approval.
RNA
Acronym for ribonucleic acid. RNA is made up of a long chain of components called nucleotides. Each nucleotide consists of a nucleobase, a ribose sugar, and a phosphate group. The sequence of nucleotides allows RNA to encode genetic information that directs the synthesis of proteins.
Second-generation biological
Improved versions of the originator medicine, which represent explicitly different products (different active ingredient, longer acting, less frequent dosing, better efficacy, higher patient convenience), are called second-generation biologicals. Unlike first-generation biologicals, these are still protected by patents.
Vaccine
A biological preparation, which is used to establish or improve immunity to a particular disease.
Similar biological medicinal product
See biosimilar medicinal product.
Acronyms – List of Abbreviations
EC – European Commission
EMA – European Medicines Agency
EU – European Union
FDA – Food and Drug Administration
IP – Intellectual property
LMWH – Low molecular weight heparins
MAA – Market Authorisation Approval
WHO – World Health Organization
Related articles
WHO definitions of biosimilars
Health Canada definitions of generics and biosimilars
FDA definitions of generics and biosimilars
EMA definitions of generics and biosimilars
Controversial nomenclature for new biosimilars
Source: www.gabionline.net
